Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakanishi, Ryuzo; Oba, Hironori; Saeki, Morihisa; Wakaida, Ikuo; Tanabe, Rie*; Ito, Yoshiro*
Optics Express (Internet), 29(4), p.5205 - 5212, 2021/02
Times Cited Count:13 Percentile:83.13(Optics)Laser-induced breakdown spectroscopy (LIBS) combined with liquid jets was applied to the detection of trace sodium (Na) in aqueous solutions. The sensitivities of two types of liquid jets were compared: a liquid cylindrical jet with a diameter of 500  m and a liquid sheet jet with a thickness of 20
m and a liquid sheet jet with a thickness of 20  m. Compared with the cylindrical jet, the liquid sheet jet effectively reduced the splash from the laser-irradiated surface and produced long-lived luminous plasma. The limit of detection (LOD) of Na was determined to be 0.57
m. Compared with the cylindrical jet, the liquid sheet jet effectively reduced the splash from the laser-irradiated surface and produced long-lived luminous plasma. The limit of detection (LOD) of Na was determined to be 0.57  g/L for the sheet jet and 10.5
g/L for the sheet jet and 10.5  g/L for the cylindrical jet. The LOD obtained for the sheet jet was comparable to those obtained for commercially available inductively coupled plasma emission spectrometers.
g/L for the cylindrical jet. The LOD obtained for the sheet jet was comparable to those obtained for commercially available inductively coupled plasma emission spectrometers.
Tamain, C.*; Bonato, L.*; Aupiais, J.*; Dumas, T.*; Guillaumont, D.*; Barkleit, A.*; Berthon, C.*; Solari, P. L.*; Ikeda, Atsushi; Guilbard, P.*; et al.
European Journal of Inorganic Chemistry, 2020(14), p.1331 - 1344, 2020/04
Times Cited Count:3 Percentile:23.3(Chemistry, Inorganic & Nuclear)The molecular characterization based on multi-technique approach has led to major highlights on revealing the coordination environment of americium (Am) surrounded by citric acid (H CitH). The structure of the different complexes at pH 1 and 3 are described. These characterizations are made possible by the comparison of the americium-citric acid system with the americium-tricarballylic acid (one analogue of the citric acid without the alpha-hydroxo group).
CitH). The structure of the different complexes at pH 1 and 3 are described. These characterizations are made possible by the comparison of the americium-citric acid system with the americium-tricarballylic acid (one analogue of the citric acid without the alpha-hydroxo group).
Yoshida, Koji*; Inoue, Takuya*; Torigoe, Motokatsu*; Yamada, Takeshi*; Shibata, Kaoru; Yamaguchi, Toshio*
Journal of Chemical Physics, 149(12), p.124502_1 - 124502_10, 2018/09
Times Cited Count:4 Percentile:17.83(Chemistry, Physical)Differential scanning calorimetry, X-ray diffraction, and quasi-elastic neutron scattering (QENS) measurements of aqueous glycine solutions confined in mesoporous silica (MCM-41) were performed at different glycine concentrations, pH, and loading ratio (= mass of glycine solution / mass of dry MCM-41) in the temperature range from 305 to 180 K to discuss the confinement effect on the thermal behavior, the structure, and the dynamic properties of the solutions.
Kido, Kentaro; Hata, Kuniki; Maruyama, Yu; Nishiyama, Yutaka; Hoshi, Harutaka*
NEA/CSNI/R(2016)5 (Internet), p.204 - 212, 2016/05
Ikeda, Takashi; Boero, M.*
Journal of Chemical Physics, 143(19), p.194510_1 - 194510_7, 2015/11
Times Cited Count:30 Percentile:74.71(Chemistry, Physical)By resorting to a novel implementation of the first-principles-based van der Waals correction based on maximally localized Wannier functions, we inspect its performance and assess its reliability for aqueous solutions of alkali metal ions. We find that van der Waals interactions, when added to the widely used revPBE gradient corrected functional, influence substantially both structural and dynamical properties of water molecules, with particular emphasis on the hydration shell of the alkali cations. These effects are more evident for strong structure-making and -breaking cationic species. Moreover, self-diffusion coefficients and reorientation correlation times of solvating water molecules change systematically, showing a trend in better agreement with experiments with respect to simulations neglecting the long-range dispersion contributions.
Nakayama, Shinichi; Sakamoto, Yoshifumi; Yamaguchi, Tetsuji; Akai, Masanobu; Tanaka, Tadao; Sato, Tsutomu*; Iida, Yoshihisa
Applied Clay Science, 27(1-2), p.53 - 65, 2004/10
Times Cited Count:83 Percentile:89.26(Chemistry, Physical)Alkaline environments induced by cement in radioactive waste repositories are likely to alter montmorillonite, the main constituent of bentonite buffer materials. Over long time periods, the alteration may cause the physical and/or chemical properties of the buffer to deteriorate. For the purpose of acquiring numerical data to quantify the effect of alteration on permeability of bentonite buffer, dissolution rates of montmorillonite and diffusivity of hydroxide ions in compacted sand-bentonite mixture specimens have been measured under highly alkaline, simulated groundwater conditions. The dissolution rate of montmorillonite was given by the linear dependence on time under the employed experimental conditions of pH 13 to 14 and temperatures of 90 to 170 C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50
C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50 C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10
C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10 to 10
to 10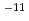 m
m /s.
/s.
Lis, S.*; Kimura, Takaumi; Yoshida, Zenko; But, S.*
Journal of Alloys and Compounds, 380(1-2), p.173 - 176, 2004/10
Times Cited Count:6 Percentile:41.12(Chemistry, Physical)no abstracts in English
Sakurai, Satoshi; Tachimori, Shoichi
Journal of Nuclear Science and Technology, 33(2), p.187 - 189, 1996/02
Times Cited Count:22 Percentile:82.8(Nuclear Science & Technology)no abstracts in English
*; *; *
JAERI-Research 94-024, 40 Pages, 1994/10
no abstracts in English
*
KURRI-TR-394, 0, p.36 - 48, 1994/07
no abstracts in English
*
JAERI-M 93-232, 41 Pages, 1993/12
no abstracts in English
Kimura, Takaumi; J.G.Serrano*; ; *; Takeishi, Hideyo
Radiochimica Acta, 58-59, p.173 - 178, 1992/00
no abstracts in English
; ; *; *; ; Machi, Sueo
J.Appl.Polym.Sci., 27, p.1033 - 1041, 1982/00
Times Cited Count:120 Percentile:97.55(Polymer Science)no abstracts in English

 in X-irradiated frozed aqueous solutions; Similar hydration shells for Cd
in X-irradiated frozed aqueous solutions; Similar hydration shells for Cd
 and Cd
and Cd
 species
speciesFujimura, Takashi; L.Kevan*
Radiation Physics and Chemistry, 19(6), p.435 - 437, 1982/00
no abstracts in English
; *; Hotta, Hiroshi;
Bulletin of the Chemical Society of Japan, 49(3), p.600 - 605, 1976/03
Times Cited Count:23no abstracts in English
 gas bubbling
gas bubbling; Tachikawa, Enzo; ;
Journal of Nuclear Science and Technology, 10(4), p.234 - 241, 1973/04
no abstracts in English
; ;
Journal of Nuclear Science and Technology, 10(2), p.95 - 100, 1973/02
no abstracts in English
Ono, Shinichi; Sasaki, Teikichi
Radioisotopes, 20(5), p.211 - 216, 1971/11
no abstracts in English
; *; *
Bulletin of the Chemical Society of Japan, 43(1), P. 294, 1970/00
Times Cited Count:1no abstracts in English
;
Journal of Inorganic and Nuclear Chemistry, 31(4), p.981 - 984, 1969/00
Times Cited Count:10no abstracts in English